RECURRENT PREGANCY LOSS (RPL)
- Home
- RECURRENT PREGANCY LOSS (RPL)
Approximately 15-20% of clinically recognized pregnancies end up in miscarriage and chromosome abnormalities are found to be the single most common cause of pregnancy loss or miscarriages (>60%).14 The exact prevalence of RPL is difficult to estimate, but most studies report that RPL affects 1-2% of women and the disease burden of the disease. In India, the prevalence of recurrent miscarriage is around 7.4%.5
At present there are many studies that have reported various etiologies in RPL patients including genetic abnormalities, uterine anatomic abnormalities, antiphospholipid syndrome, endocrine abnormalities, infections, hormonal or metabolic disorders, sperm quality and life style related disorders (Figure 1). At present diagnosis and management of RPL can be done only in 50% of patient while remaining patients had to be categorized under false idiopathic category with no diagnosis.
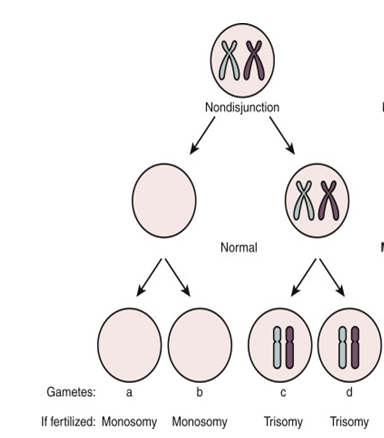
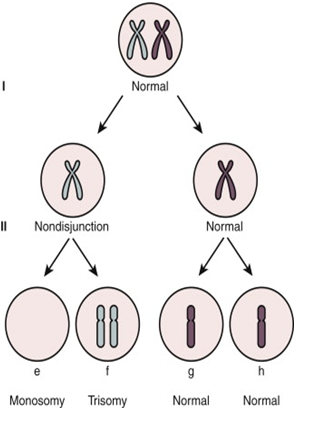
Figure 1: Diagrammatic representation of mechanism of Chromosome Aneuploidy due to segregation error or non-disjunction of meiotic chromosomes (Meiosis I & Meiosis II) one of the most common cause of aneuploidy during Germ cells (egg & sperm) development .
Pregnancy loss can be any of the following different type of chromosome abnormalities The most common etiologies include whole chromosome aneuploidies including monosomy X, trisomy for chromosome 16 & 22.
Different Types Chromosome Abnormalities observed in Pregnancy Loss (POC)
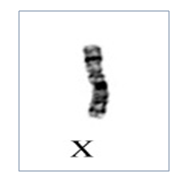
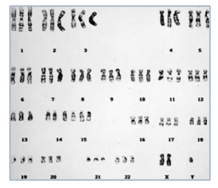
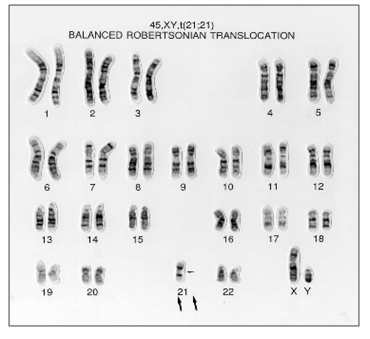
There are two types of Balanced translocations that are commonly observed in 2-5% of couples with recurrent miscarriages and needs to be rule out before planning the future pregnancy.
Robertsonian translocations are the most common types of structural chromosome rearrangements, involving the acrocentric chromosomes (chr. 13, 14, 15, 21 22) in humans. Genetically balanced carriers of these translocations have an increased incidence of infertility as well as a risk for genetic imbalances among their offspring. The risk of Down syndrome (trisomy 21) and Patau syndrome (trisomy 13) is elevated in the offspring of the rob (14;21) and the rob (13;14) balanced carriers, respectively.
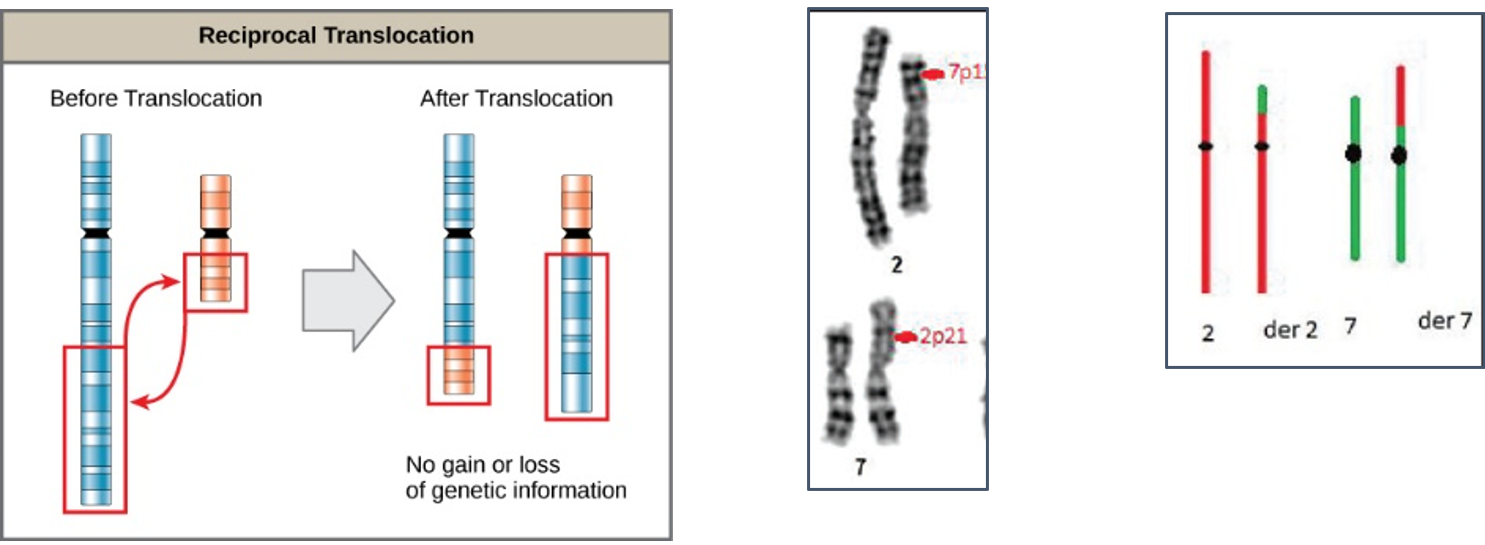
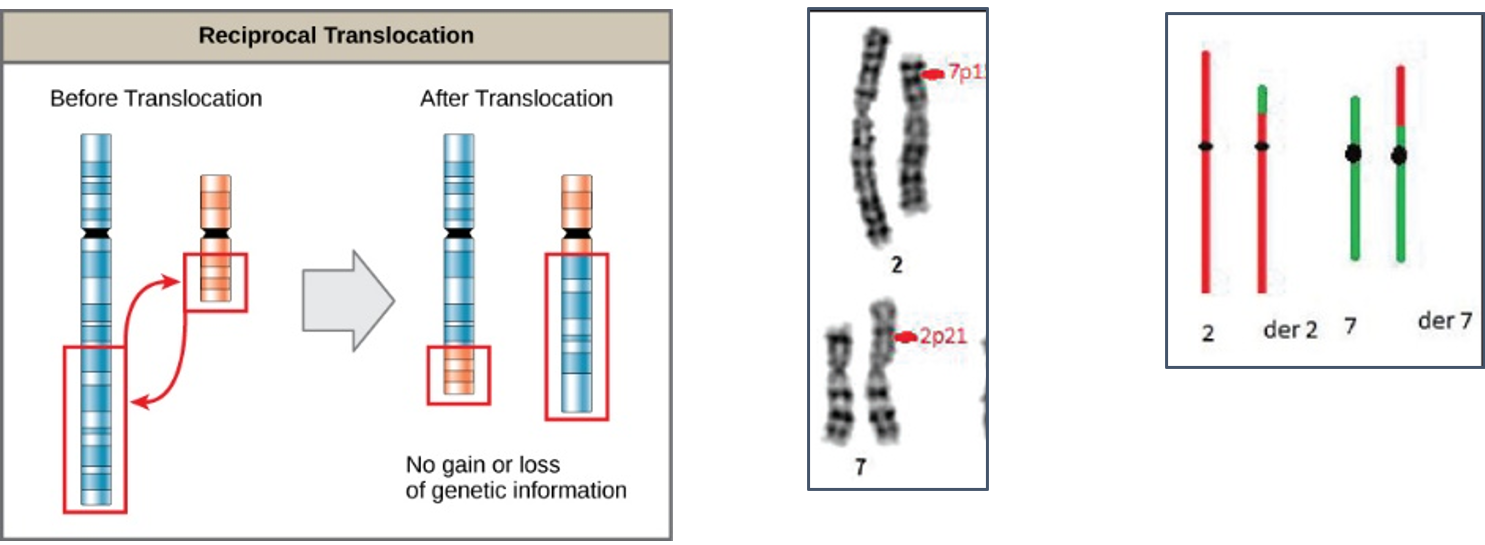
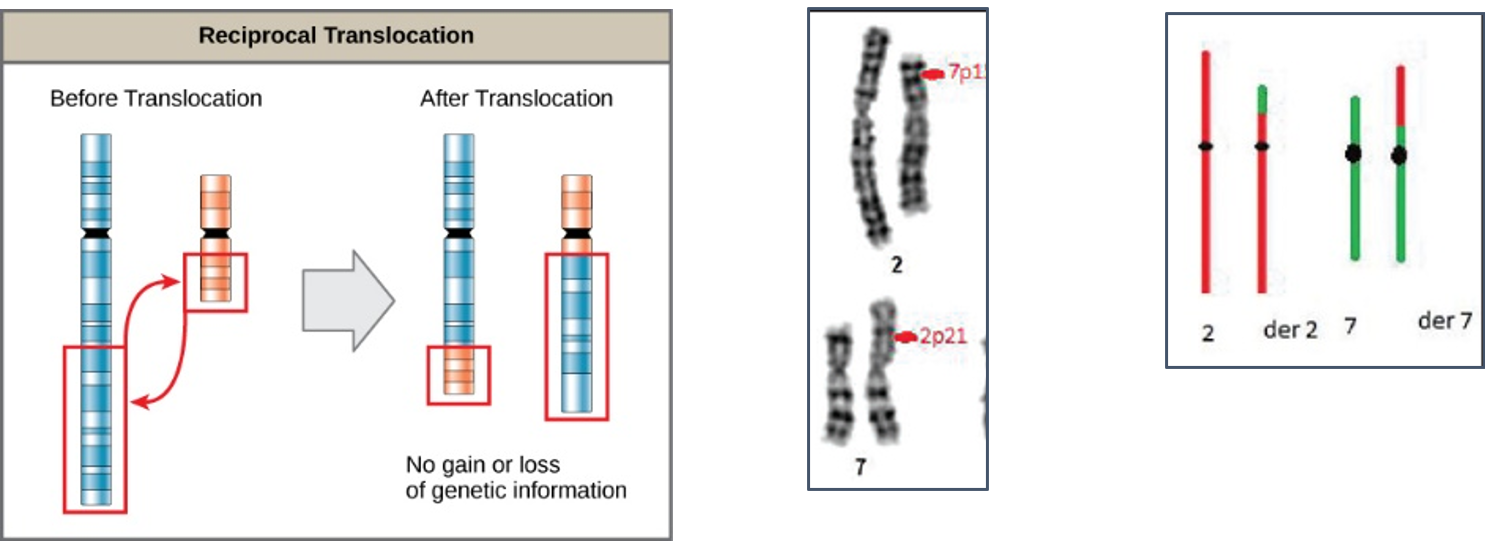
Reciprocal translocations are defined as exchange of genetic material between homologous chromosomes. These are most commonly balanced exchanges, such that no genetic material is lost and individuals are absolutely phenotypically normal. Patients with reciprocal translocation may have a normal phenotype, but this chromosomal abnormality can result in abnormal meiosis during gamete formation in sperms in male or egg (ovum in women. The resulting sperm are more likely to have aneuploidy, which can manifest
with recurrent pregnancy loss.
Reciprocal translocations are the most common structural chromosome abnormality in the general population (0.2% of newborns) commonly observed in infertile men are even more likely to have this abnormality (0.6%). Also observed in different type of leukemia (blood cancer) commonly in chronic myeloid leukemia (CML).
RECOMMENDATIONS FROM PROFESSIONAL BODIES
American Society of Reproductive Medicine (ASRM)
The American College of Obstetrician and Gynecologists:

European Society of Human Reproduction & Embryology (ESHRE):




TABLE 1: Summary of current recommendations from different professional bodies for genetic evaluation of Recurrent Pregnancy Loss (RPL) patient’s
| Professional | Current Recommendations | Evidence |
|---|---|---|
| ESHRE* | Array based comparative genomic hybridization (array-CGH) or microarray is recommended based on a reduction in maternal cell contamination. | Strong |
| ESHRE* | Genetic analysis of pregnancy tissue though is not routinely recommended in RPL but it could be performed for explanatory purposes. | Conditional |
| ESHRE* | Parental Karyotyping at present not routinely recommended in couple with RPL, but it could be carried out after individuals risk assessment. | Conditional |
| ASRM* | Peripheral karyotyping is preferred for parents for detection of balanced structural chromosome abnormalities. | Strong |
| ASRM* | Karyotype analysis of POC may be useful in the setting of ongoing therapy of RPL but there is possibility of maternal tissue contamination in the specimen | Conditional |
| ACOG/SMFM* | In cases of intra uterine fetal demise or still birth further cytogenetic analysis is desired, Chromosomal microarray analysis on the fetal tissue (i.e. amniotic fluid, placenta, or products of conception) is recommended in the evaluation with increased likelihood of obtaining results and improved detection of causative abnormalities | Recommended |
ESHRE: European Society of Human Reproduction & Embryology; ASRM: American Society of Reproductive Medicine; ACOG: The American College of Obstetricians and Gynecologists, SMFM: Society of Maternal Fetal Medicine.
One of the latest study identified probable cause of pregnancy loss in majority of RPL patients by evaluation of POC through 24-chromosome pair microarray along with standard RPL workup by ASRM guidelines11. It was concluded that evaluation of POC using 24-chromosome microarray analysis adds significantly to the existing ASRM guidelines recommended for RPL evaluation. It was also concluded that all couples with RPL should be offered genetic evaluation on miscarriage tissue obtained at the time of the second and subsequent pregnancy losses. There was a testing algorithm proposed in combination of a genetic evaluation that will identify a probable or definitive cause of RPL in over 90% of miscarriages (Figure 2).
TABLE 2: Comparison between some of the common technologies used for examination of embryonic/fetal material (product of conception)
| Method Characteristics (Technique) | Microarray (Comparative Genomic Hybridization) | Karyotype (Conventional Culture technique) | FISH (Fluorescence in-situ Hybridization) | QF-PCR (Quantitative Fluorescent- Polymerase chain Reaction) | NGS (Next Generation Sequencing) |
|---|---|---|---|---|---|
| Detect | Chromosome abnormalities (aneuploidies, triplody),Unbalanced structural changes (duplication, deletion, amplification | Changes is chromosome number (aneuploidies, polyploidy) Structural Abnormalities (balanced & unbalanced translocation) | Chromosome aneuploidies Diagnosis of sub-microscopic chromosome aberration, Structural translocation | Detect aneuploidies for chromosome 13, 18, 21, X, Y, 15, 16 and 22 | Sequencing of large genomic regions, high number of genes with high throughput |
| Samples Type | Fresh Tissue, FFPE Block | Fresh Tissue, culture cells | Fresh Tissue, uncultured (interphase) cells | Fresh Tissue/ DNA | Fresh/ FFPE |
| Limitation | Cannot detect unbalanced translocation & low level of mosaicism (<10%), | The requirement of culture of cells, (high culture failure rate 10-20%) | Diagnosis specific and limited to probes utilized in kit | Diagnosis within intended use of kit only | |
| Culture Failures | No | Yes | No | No | No |
| Diagnostic Yield | High (100-400 kb) | Low (5- 10 MB) | Moderate (100–200 Kb) | Moderate | Very High (<50Kb) |
| Maternal Cell Contamination (MCC) | No | Yes | Yes | No | No |
| Turn Around Time (TAT) for getting results | 10-12 days | 14-21 days | 24-48 hours | < 24 hours | 21-28 days |
| Recommended by Guidelines | Yes | Yes | No | No | No |
| Cost (INR) | 15000-18000 | 7000-8000 | 6000-7000 | 4000-6000 | 20000-25000 |
Table 3: Lists of Investigations for diagnosis and further management of recurrent pregnancy loss patients
| Test Code | Test Name | Specimen Requirement | Sample Type | Specimen Stability | Transport Condition | Cost (INR) | Turn Around Time (TAT) Reports |
|---|---|---|---|---|---|---|---|
| KPB001 | Couple Karyotype- (Both Husband & Wife) | 2-4 ml Blood in Na-Heparin (Green Top) vial | Peripheral Blood sample | Within 48-72 hours from time of collection | Ambient Temperature with cool pack (2-8˚C) | 4500 | 10-15 days |
| MPOC02 | Microarray- Product of Conception (POC) (315K) | Placental/ fetal tissue (POC) in normal saline or transport medium (request lab) in sterile container | 25-50 mg placental tissue/ villus of fetal origin or skin (3 mm) with no maternal tissue contamination | Within 24 hours from time of collection | Ambient Temperature with cool pack (2-8˚C) | 14000 | 10-15 days |
| KPOC03 | Karyotype/Fluorescence in-situ Hybridization (FISH) - Product of Conception (POC) | Placental/ fetal tissue (POC) in normal saline or transport medium (request lab) in sterile container | 25-50 mg placental tissue/ villus of fetal origin or skin (3 mm) with no maternal tissue contamination | Within 24 hours from time of collection | Ambient Temperature with cool pack (2-8˚C) | 6000 | 07-21 days |
| MAPS04 | Antiphospholipid Syndrome 1. Lupus Anticoagulant 2. Anticardiolipin Antibody (IgG & IgM) 3. Anti- β2 glycoprotein (IgG &IgM) | Plain Serum (Red Top) vial (2-4 ml) Sodium Citrate (Blue Top) vial (2-4 ml | 2-4 ml Peripheral Whole Blood | Within 24 hours from time of collection | Ambient Temperature with cool pack (2-8˚C) | 4500 | 07 days |
| MITP05 | Inherited Thrombophilia Profile 1. Factor V Leiden mutation (R506Q/G1691A, T138C) 2. Factor II /Prothrombin Gene mutation (G20210A) | Whole Blood EDTA (Purple Top) Vial (2-4 ml) | 2-4 ml Peripheral Whole Blood | Within 24-72 hours from time of collection | Ambient Temperature with cool pack (2-8˚C) | 12500 | 15-21 days |
| MMTH06 | MTHFR gene two common mutation (A222V/C677T+E429A/1298C) | Whole Blood EDTA (Purple Top) Vial (2-4 ml) | 2-4 ml Peripheral Whole Blood | Within 24-72 hours from time of collection | Ambient Temperature with cool pack (2-8˚C) | 7000 | 07 days |
| FEPAT07 | Fetal Autopsy with Placental Pathology Studies | Fetus & Placental/ fetal tissues in normal saline in sterile container with Signed Consent form | Fetal Tissues/ Product of Conception | Within 24 hours from time of collection | Room Temperature (Transfer between (20-25˚C) | 12000 | 03-04 weeks |
| GECOU08 | Genetic Counselling | NA* | NA | NA | NA | 600 | 60 mins |