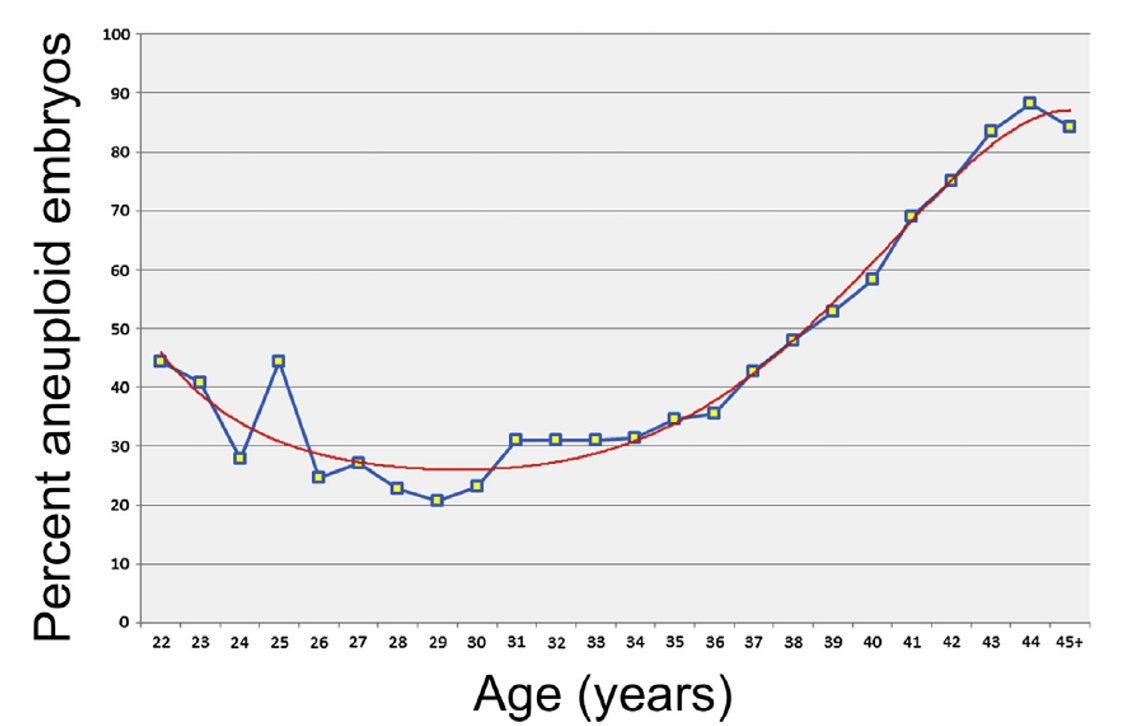
Chromosome abnormalities occur in approximately 1 in 150 live births (1), the prevalence of chromosome abnormalities is greater earlier in gestations accounting for large proportion of pregnancy loss. The incidence of fetal chromosomal abnormalities (aneuploidy) increases as a women age increase (Figure 1) but can affect patients of any age and is not related to race or ethnicity.
Trisomy 21 (Down Syndrome) is the most common autosomal chromosomal aneuploidy in live born infants with a prevalence of approximately 1 in 700 live births reported worldwide. Due to the high birth in India almost half a million babies are born annually with malformations; the figure for Down syndrome (trisomy 21) is 21,000 (21) or 1 per 1150 births (2). Aneuploidies of five particular chromosomes (13, 18, 21, X, Y) account for 80-95% clinically relevant cytogenetics abnormalities that lead to infants born with congenital defects.
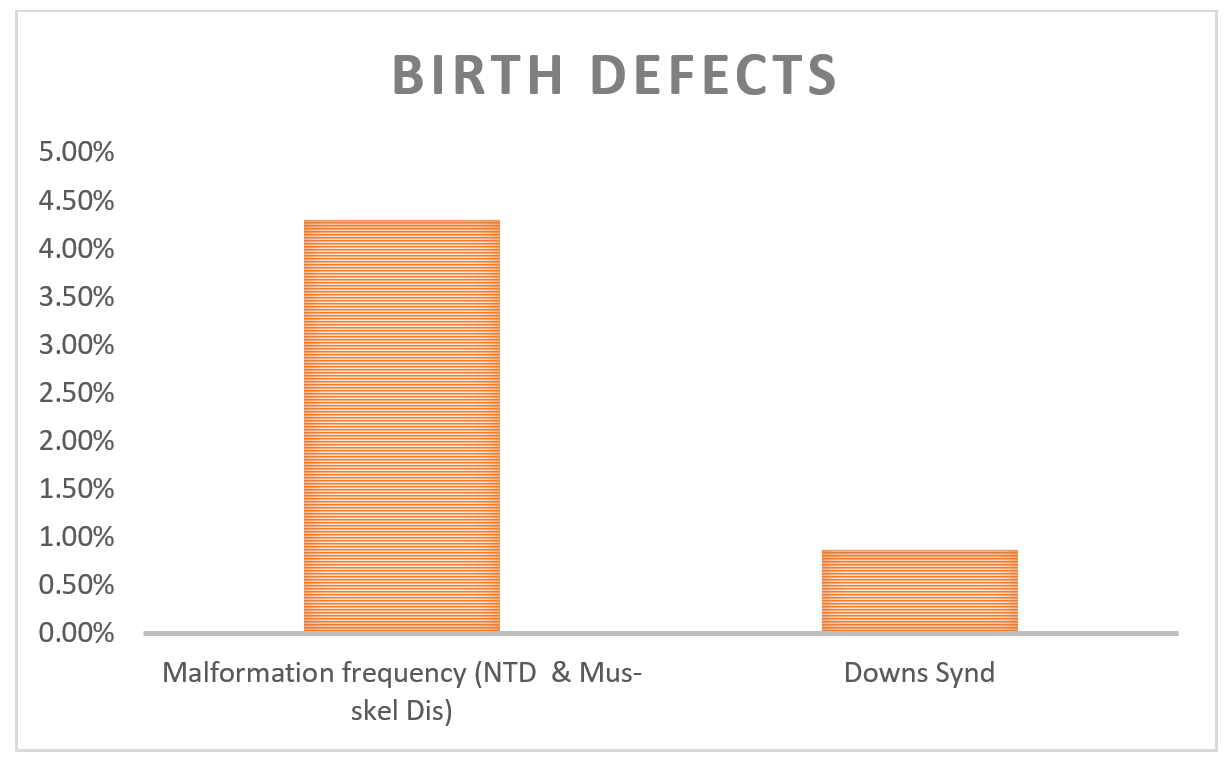
Figure 2: Observation from Study carried out at three centers (Mumbai, Delhi and Baroda) on 94,610 newborns (Verma IC et al., 2000)

As per this ACOG practice bulletin “Testing for chromosomal abnormalities should be informed patient choice based on provision of adequate and accurate information, and the patient’s clinical context, accessible health care resources, values, interests and goals. Prenatal genetic screening (serum screening Double/Quadruple marker with or without nuchal translucency [NT] ultrasound or cell free DNA screening/ NIPT) and diagnostic testing (CVS or amniocentesis) option should be discussed and offered to all pregnancy patients regardless of age or risk for chromosomal abnormality. After review and discussion, every patient has the right to pursue or decline prenatal genetic screening and diagnostic testing”.
The most commonly practiced approach in India is single time point screening approaches that include first trimester screening along with measurement of
Table 1: Characteristic, Advantage and Disadvantage of Common Screening Tests for Chromosome Abnormalities along with test summary
| Screening Test) | Gestation Age for Screening (weeks) | Detection Rate (DR) for Trisomy 21 (%) | False/Screen Positive Rate (%) | Advantage | Disadvantage | Methodology | Sample Type | Cost (INR) | Turn Around Time (TAT) Reports | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cell free Fetal DNA (NIPT) | 9-10 weeks till term# | 99% | 2-4% | Highest Detection Rate; Can be performed as early as 9-10 weeks gestation | Result may reflect false positive due to underlying maternal aneuploidy or maternal disease | Next Generation Sequencing (massively parallel, MPSS/SNP) of Cell free DNA from dead trophoblast cells in maternal blood | Maternal Blood in Special Streck (Black) Tube | 15000 | 07-10 days | |
| Double Marker Test/ First Trimester Screen | 10-13 6/7 weeks | 82-87% | 5% | Early screening; single time point test | Lower detection rate with first and second trimester combined NT required | Serum Analytes for Pregnancy-associated plasma protein (PAPP-A), free beta (β) hCG | Mother Blood (Serum) in Plain (Red) Tube | 2400 | 24-48 hours | |
| Quadruple(Quad) Marker Test | 15-22 weeks | 81% | 5% | Early screening; single time point test | Lower detection rate than first trimester and first and second trimester combined tests | Serum Analytes for hCG; AFP (Alpha fetoprotein); unconjugated estriol (uE3); Inhibin A (DIA) | Mother Blood (Serum) in Plain (Red) Tube | 3800 | 24-48 hours | |
| Integrated Screen | 10-13 6/7 then 15-22 weeks | 96% | 5% | High DR | Two samples needed No first trimester results NT required | NT + PAPPA-A, then quad screen | Mother Blood (Serum) in Plain (Red) Tube | 10-12K | 24-48 hours |